
The “Egg in a Bottle” experiment is one of the most fascinating and visually striking science demonstrations. It appears almost magical—how does a hard-boiled egg, larger than the neck of a bottle, get sucked inside without being touched? At first glance, it defies logic. But behind the illusion lies a brilliant application of physical science, particularly air pressure, temperature, and gas laws.
This simple yet powerful demonstration is a classic in classrooms and science fairs, not just because of its wow factor but because it teaches core scientific principles in a hands-on, memorable way. In this article, we’ll explore how the Egg in a Bottle experiment works, the science behind it, and how you can try it yourself safely.
What You Need for the Egg in a Bottle Experiment
Before diving into the scientific explanation, let’s look at the materials typically used:
Materials:
- A hard-boiled egg (peeled)
- A glass bottle with a neck slightly narrower than the egg (an old-fashioned milk bottle or Erlenmeyer flask works well)
- Matches or a lighter
- Strips of paper or a small piece of burning material (like a paper towel)
- Tongs or tweezers (for safety)
- Cold water (optional for variation)
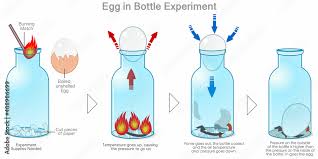
The Setup
- Boil and peel the egg, making sure it’s slightly larger than the mouth of the bottle.
- Place the bottle upright on a table.
- Light a small piece of paper and drop it into the bottle quickly.
- Immediately place the egg on the mouth of the bottle.
Within a few seconds, the egg jiggles slightly and is suddenly sucked into the bottle, seemingly without force or manipulation.
Why It Happens – The Science Explained
To understand this classic demonstration, we need to explore three scientific concepts:
- Air pressure
- Gas laws (specifically Charles’s Law and Boyle’s Law)
- Thermal contraction
Let’s break it down step-by-step.
Step 1: Sealing the Bottle with an Egg
When you place the egg on top of the bottle, you’re effectively sealing the opening. The bottle now has a limited air supply and any changes inside the bottle will directly affect the egg.
Step 2: Burning the Paper
The lit paper burns quickly and heats the air inside the bottle. This heat causes the air molecules to move faster and spread apart, increasing the internal pressure and pushing some air out of the bottle. You may even see the egg wiggle slightly as some air escapes past it.
This process follows Charles’s Law, which states:
“At constant pressure, the volume of a gas increases with temperature.”
However, since the volume of the bottle is fixed, the gas expands and escapes out of the bottle while burning.
Step 3: The Flame Goes Out
After a few seconds, the flame consumes all the available oxygen in the bottle and goes out. At this point, the temperature inside begins to drop rapidly.
As the air inside the bottle cools down, the gas molecules slow down and take up less space. This reduces the pressure inside the bottle.
According to Boyle’s Law:
“For a fixed amount of gas at constant temperature, the pressure and volume are inversely related.”
While the volume stays constant, a decrease in temperature causes a decrease in pressure. The pressure outside the bottle (atmospheric pressure) is now greater than the pressure inside.
Step 4: The Egg is Pushed In
The external atmospheric pressure, which is stronger than the pressure inside the bottle, pushes the egg into the bottle to equalize the pressure difference.
It’s not that the egg is “sucked in” by vacuum—it’s actually pushed in by the air outside, exerting a force on the egg.
This force is surprisingly strong. At sea level, atmospheric pressure is approximately 14.7 pounds per square inch (psi), meaning every square inch of the egg has 14.7 pounds of air pressing on it. If the egg fits snugly over the bottle’s opening, the atmospheric pressure over the whole egg can easily be strong enough to force it through the narrow opening.
Understanding the Physics
Let’s look at the physical laws in action:
1. Charles’s Law
This law explains what happens when gas is heated or cooled at constant pressure.
Formula:
V1/T1=V2/T2V_1 / T_1 = V_2 / T_2V1/T1=V2/T2
As the flame heats the air inside the bottle, the volume increases (some escapes). When the flame goes out, temperature drops, volume decreases, and pressure inside the bottle drops.
2. Boyle’s Law
This law states that for a fixed temperature, the pressure and volume of a gas are inversely proportional.
Formula:
P1×V1=P2×V2P_1 \times V_1 = P_2 \times V_2P1×V1=P2×V2
Here, pressure inside drops as temperature drops, while volume is constant. So, the external pressure wins, pushing the egg inward.
3. Atmospheric Pressure
Atmospheric pressure is the “silent force” acting all the time. You don’t usually feel it because it’s balanced by internal pressure. But once there’s a pressure imbalance, the effects can be dramatic—as seen in this experiment.
Can the Egg Be Removed?
Yes, and the reversal is just as fun. To remove the egg without breaking it:
- Turn the bottle upside down.
- Hold it carefully so the egg rests near the opening.
- Blow hard into the bottle.
- Quickly turn the bottle upright again.
This increases the air pressure inside the bottle. The higher internal pressure pushes the egg back out—again, illustrating the power of air pressure.
Variations of the Experiment
1. Ice Instead of Fire
After sealing the egg on the bottle, place ice around the bottle’s neck. The cold causes the air inside to contract, reducing pressure and causing the egg to be pushed in—without fire.
2. Hot Water and Cold Bottle
Pour hot water into the bottle, swirl it around, then dump it out. Immediately place the egg on top. The hot air inside cools and contracts, sucking the egg in.
3. Invert the Setup
Place the egg inside the bottle, then heat the bottom. As the air expands, pressure builds. If the egg is small enough or the pressure high enough, it may be pushed out when the heat is removed—demonstrating reversible pressure systems.
Safety Tips
- Adult supervision is essential, especially when using fire.
- Use tongs or tweezers to insert burning paper.
- Don’t use plastic bottles—they can melt or release fumes.
- Perform in a well-ventilated area and keep water nearby for safety.
Educational Value
The Egg in a Bottle experiment is more than just a neat trick. It teaches several core concepts:
- Gas expansion and contraction with temperature changes
- Air pressure and atmospheric force
- The role of temperature in pressure systems
- Newton’s laws of motion (when the egg moves)
- Thermodynamic principles
This makes it an ideal experiment for:
- Physics and chemistry classes
- Science fairs
- STEM outreach
- Homeschooling activities
Real-World Applications
Understanding pressure differentials is critical in many real-world systems:
- Aircraft cabins: Pressurized to keep passengers safe at high altitudes.
- Syringes and vacuums: Use pressure to move fluids.
- Engines: Internal combustion relies on pressure differences to produce motion.
- Weather systems: High and low pressure influence wind and storms.
By grasping the Egg in a Bottle demonstration, students get a window into these more complex systems.
Conclusion
The Egg in a Bottle experiment is a wonderful example of how simple materials can reveal complex scientific truths. It captivates observers with its seemingly impossible result and introduces key principles of physics in a clear, engaging way.
What looks like magic is actually a beautiful illustration of air pressure, temperature changes, and gas laws in action. It reminds us that science is not just theoretical—it’s visible, tactile, and all around us, even in the kitchen.
Whether you’re a student, teacher, or curious experimenter, this demonstration provides a perfect blend of fun and fundamental physics. So the next time you boil an egg, consider putting science to the test—and maybe even blow a few minds.
Let me know if you want a diagram or printable handout for classroom use!


